AFM in Virus Research
How AFM (Atomic Force Microscopy) can be used to study viruses, including measuring biomechanical properties and virus-cell receptor interactions.
Blue Scientific is the official distributor for Bruker JPK BioAFM instruments in Norway, Sweden, Denmark, Finland and Iceland. For more information or quotes, please get in touch.
Bruker JPK BioAFM range
Bruker application note
Contact us on +44 (0)1223 422 269 or info@blue-scientific.com
AFM Applications in Virology
AFM (Atomic Force Microscopy) is a key technique for imaging of biological samples in liquid with nanometre resolution, ideal for investigating viruses.
This article explains how AFM can be used to examine various types of viruses:
- High-resolution topography imaging
- Measuring the mechanical properties of viruses
- Interactions of with and adhesion to cells
- Electrical properties
- … and more
Further details about the examples mentioned are available in an application note from Bruker.
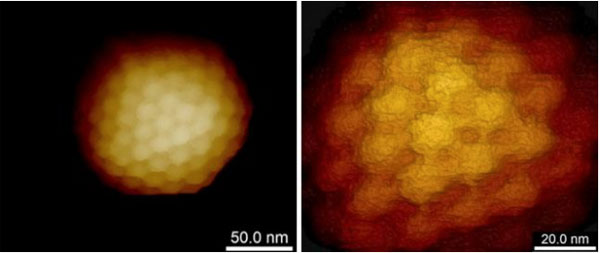
High Resolution Imaging
AFM can visualise individual virions in a liquid environment. For best results, the sample must be immobilised. Protocols for molecule immobilisation can be used on flat surfaces, or the surface can be coated with adhesion molecules, or hydrophobic surfaces work for some viruses. An atomically flat substrate is not required for imaging and in many cases regular glass is sufficient.
Clusters or 2D crystals of viruses can be easiest to image at higher resolution. The individual virions support each other and restrict lateral motion.
The temperature can also be varied, for studying temperature induced degradation. Recently AFM was used by Sharma et al. to demonstrate the effects of mildly elevated temperatures on SARS-CoV-2 virus-like particles in wet and dry conditions (article published in BBRC).
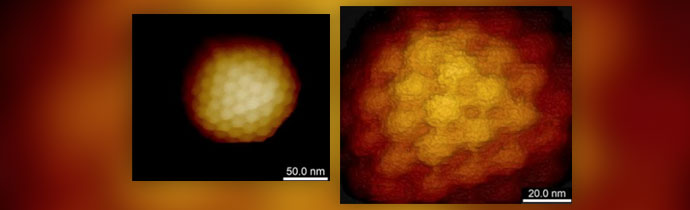
AFM Modes for Virus Visualisation
Despite being robust in general, viruses can be sensitive to in vitro AFM imaging. It’s important to use extremely low imaging forces to resolve fine structural details such as proteins or capsomers on the surface.
Several AFM modes are available from Bruker, which dramatically improve results for high resolution imaging in liquids, and also make the process much easier:
- Tapping mode – Enables imaging of samples too fragile to withstand the lateral forces of contact mode, with scan speeds much higher than non-contact mode.
- PeakForce Tapping (PFT) mode – Extremely high resolution imaging, opening up applications not previously possible. Nanoscale properties can be mapped simultaneously alongside imaging. More details…
- Quantitative Imaging (QI) mode – Makes it easier to reach the lowest peak forces down to 10pN, ideal for soft, sticky, brittle or loosely attached specimens.
If you’d like more details about these modes, application notes are available – please contact us.
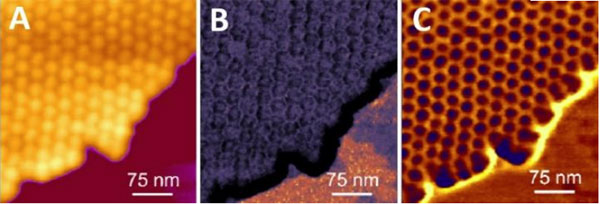
Virus Uptake and Release
AFM can visualise both the virus and its host cell in their native physiological conditions. For live samples the resolution can be down to 50- 100 nm and on fixed specimens down to 20-50 nm.
The process of virus uptake and release can be studied in vitro. By adjusting the forces, you can image structures on the surface or the cytoskeleton and structures beneath the plasma membrane.
Bruker’s PeakForce Tapping and QI modes record force-distance curves at every pixel so the topography can be reconstructed at zero force, regardless of the forces used. This enables you to examine:
- Viruses attached to the cell membrane
- Partially internalised viruses
- Viruses inside cells that have just been internalised or are about to be released
With the Bruker JPK NanoWizardULTRA Speed 2, virus uptake and release can be imaged at 10 frames per second.
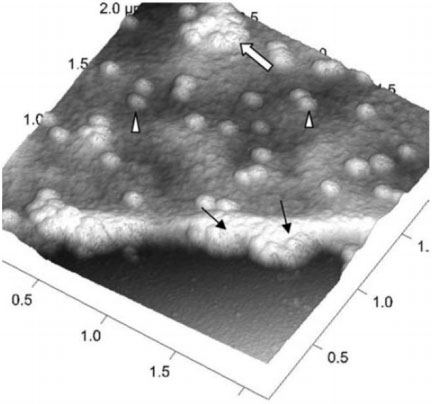
Mechanical Properties
With Bruker JPK AFM you can perform mechanical testing, applying load in the range of pN to µN with nanometre spatial resolution. This enables studies of the correlation between virus stiffness and ability to enter target cells, and how this changes as the virus matures.
Modes such as single-point ramping or spectroscopy on a single viral capsid can be complemented with the simultaneous imaging and mapping of mechanical properties, using PeakForce Quantitative Nanomechanical Mapping (PF-QNM) and QI modes. After acquiring the AFM image, the probe is positioned precisely on top of the individual capsid for indentation measurements.
Adhesion to Host Cells
Absorption to the host cell can take place in various ways, with a structure on the virus recognising a structure on the host cell to bind to. This might be a spike and enzyme receptor, for example. AFM is ideal for characterising these individual virus binding events to host cells at high temporal and spatial resolution.
Probes are available from Bruker that are specifically designed for imaging and mapping the mechanical properties of live cells.
Simultaneous Adhesion and Mechanical Mapping
This data can even be collected simultaneously, to map the mechanical properties of viruses and measure the binding kinetics to the host cell. This information can then be correlated, for further insights.
Bruker’s PF-QNM and QI modes both generate force curve-based images, from which you can calculate tip-sample adhesion and mechanical properties.
Electrical Measurements
Another valuable feature of AFM is the ability to measure the electrical properties of materials at high spatial resolution.
Bruker’s low force, high-resolution imaging PeakForce Tapping mode can be combined with multiple electrical characterisation techniques, which has been used in numerous scientific publications.
Several modes are available that enable simultaneous acquisition of mechanical and electrical properties on samples that would previously have been very challenging, including soft polymers and organic photovoltaic materials:
- PeakForce TUNA
- PeakForce KPFM
- PeakForce sMIM
- PeakForce SECM
- DataCube
Please contact us if you’d like to know more about what’s possible with these modes and how they work.
Bruker JPK BioAFM
We offer the full range of Bruker JPK instruments, including:
- AFM
- Automated force spectroscopy
- Optical tweezers and optical trapping
- Cell/tissue mechanics and adhesion
Further Information
Further details about these techniques are available in an application note from Bruker.
Application note: Investigation of Viruses Using Atomic Force Microscopy
We’re available to answer all your questions and advise on which modes and techniques would work best for your research. Blue Scientific is the official distributor of Bruker JPK BioAFM systems in the Nordic region (Finland, Norway, Sweden, Denmark and Iceland).